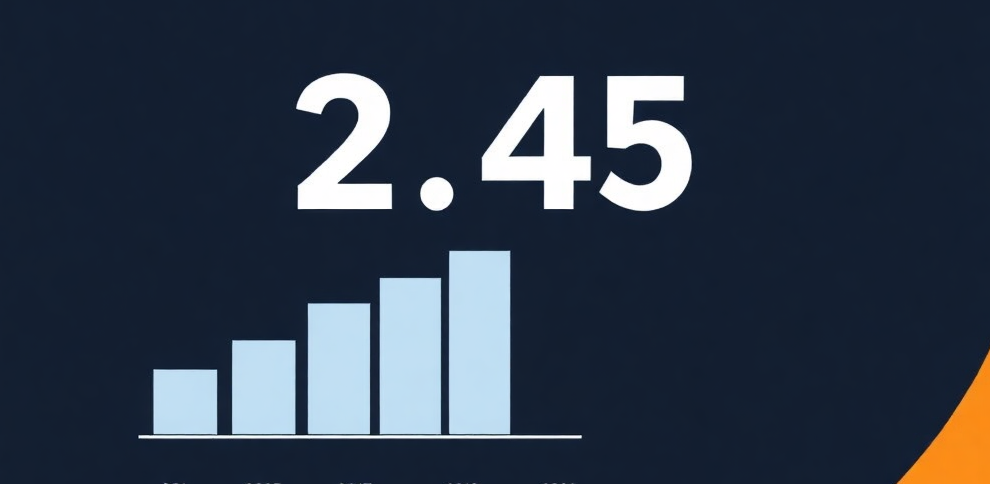
The clinical trial imaging market has emerged as one of the most significant sectors in the healthcare industry, fueled by a surge in clinical trials and advancements in imaging technologies. By 2032, the global clinical trial imaging market is expected to reach USD 2.45 billion, growing at a compound annual growth rate (CAGR) of 7.86%. This market’s growth is largely driven by increasing demand for high-precision imaging technologies in clinical trials across various therapeutic areas like oncology, neurology, and cardiology.
Clinical trials have become essential for testing new drugs and therapies, and imaging has played a critical role in monitoring treatment outcomes, safety, and disease progression. With the rise of personalized medicine, imaging technologies are helping tailor treatments to individual patients, making clinical trials more efficient and accurate. This article explores the growth trajectory, technological advancements, market drivers, and challenges of the clinical trial imaging industry.
Market Overview
The clinical trial imaging market is witnessing significant expansion due to the increasing prevalence of chronic diseases, the rising number of clinical trials, and technological advancements in imaging techniques. The use of imaging in clinical trials has enabled the healthcare industry to achieve better insights into how drugs work, how they affect different tissues, and the overall safety and efficacy of the treatments being tested.
Clinical trial imaging encompasses various modalities such as Magnetic Resonance Imaging (MRI), Computed Tomography (CT), Positron Emission Tomography (PET), Ultrasound, and other advanced imaging technologies. These imaging techniques allow researchers and clinicians to observe and measure changes in tissues, organs, and systems without the need for invasive procedures.
Factors Driving the Market
1. Increase in Clinical Trials
The growth of the global clinical trials market is one of the most significant drivers of the clinical trial imaging sector. Over 450,000 clinical trials were registered globally by the end of 2023, according to ClinicalTrials.gov, marking an increase in the number of trials conducted worldwide. The rise in the number of trials is primarily driven by the expanding global demand for new drugs, personalized therapies, and more effective treatments for various diseases.
Clinical trial imaging is becoming increasingly important in this context, as it helps researchers visualize and evaluate how a drug interacts with the body. These imaging technologies provide detailed insights into a patient’s response to a drug, including any side effects, disease progression, or therapeutic benefits. This ability to monitor patients non-invasively throughout the trial is crucial for advancing clinical research.
2. Technological Advancements in Imaging
The rapid evolution of imaging technologies is another crucial factor driving the growth of the clinical trial imaging market. New innovations in MRI, CT, and PET scans have significantly enhanced their accuracy, sensitivity, and resolution. These advancements allow researchers to gather more precise data, leading to more reliable and efficient clinical trials.
Additionally, the integration of Artificial Intelligence (AI) and Machine Learning (ML) into imaging analysis has revolutionized how data is processed. AI-powered imaging software can detect subtle patterns in imaging data that might not be visible to the human eye, improving diagnostic accuracy and helping researchers make more informed decisions.
3. Growing Prevalence of Chronic Diseases
The global rise in chronic diseases such as cancer, neurological disorders, cardiovascular diseases, and diabetes has prompted an increase in clinical trials aimed at developing effective treatments. In oncology, for example, clinical trials often rely heavily on imaging technologies like CT, MRI, and PET scans to monitor tumor growth, detect metastasis, and assess the effectiveness of cancer therapies.
With a greater focus on treating chronic diseases, clinical trials require more advanced imaging modalities to provide accurate, real-time insights into how treatments affect the body. This shift toward personalized treatments further drives demand for imaging technologies that can capture and analyze detailed physiological data.
4. Rise of Personalized Medicine
Personalized medicine, which tailors medical treatment to individual patient characteristics, has become one of the most significant trends in the healthcare industry. This approach requires highly accurate, individualized data to assess how a patient is responding to a specific treatment. Imaging technologies play a critical role in this process by providing detailed insights into how drugs interact with different tissues in the body.
As personalized medicine continues to grow in popularity, clinical trial imaging is increasingly being used to identify which treatments work best for specific patient populations. This trend is expected to drive further growth in the clinical trial imaging market as more therapies are developed with a personalized approach.
5. Support from Regulatory Bodies
Regulatory agencies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have recognized the importance of clinical trial imaging and its role in assessing the safety and efficacy of new drugs. These regulatory bodies are actively developing guidelines for incorporating imaging data into clinical trials and drug approval processes. As a result, pharmaceutical companies and contract research organizations (CROs) are increasingly adopting clinical trial imaging solutions to meet regulatory standards and expedite the drug approval process.
Technological Advancements in Clinical Trial Imaging
The clinical trial imaging market has benefited immensely from technological innovations that have improved the accuracy, speed, and versatility of imaging modalities. Here are some of the key technologies driving the industry forward:
1. Magnetic Resonance Imaging (MRI)
MRI has long been an essential imaging tool in clinical trials, particularly in the fields of neurology and oncology. The recent advent of functional MRI (fMRI) has expanded MRI’s capabilities, allowing researchers to observe brain activity in response to drug treatment. This is particularly valuable for clinical trials investigating neurological disorders such as Alzheimer’s disease and multiple sclerosis.
Additionally, Diffusion Tensor Imaging (DTI), a specialized MRI technique, helps researchers evaluate the integrity of white matter in the brain. This is useful in neurological clinical trials to monitor the effects of drugs on brain function and disease progression.
2. Computed Tomography (CT)
CT scans provide high-resolution images of the body’s internal structures and are especially useful in cancer trials to assess tumor size, detect metastasis, and evaluate the effects of chemotherapy. CT scans are also employed in cardiovascular clinical trials to measure changes in blood vessels and heart function.
Recent advancements in CT technology, such as the integration of artificial intelligence for faster data analysis, have significantly improved its role in clinical trials. AI algorithms can automatically detect and interpret abnormalities in CT scans, enabling faster decision-making and more accurate results.
3. Positron Emission Tomography (PET)
PET imaging is particularly valuable in oncology trials, where it helps track the metabolic activity of tumors and monitor the effects of treatments. PET scans allow for the detection of cancer at an early stage by identifying areas of high metabolic activity, a hallmark of cancer cells. The development of new radiotracers has further enhanced the role of PET in clinical trials, providing more detailed and specific insights into the behavior of cancer cells.
PET scans are also increasingly being used in neurology trials, where they allow researchers to track changes in the brain that result from neurological disorders or treatment interventions.
4. Ultrasound Imaging
Ultrasound technology is gaining popularity in clinical trials due to its non-invasive nature, real-time imaging, and relatively low cost. It is commonly used in cardiovascular and musculoskeletal clinical trials to monitor blood flow, assess tissue damage, and evaluate the effects of treatments on joints and muscles. Recent advancements in high-frequency ultrasound technology have significantly improved image resolution, making it a valuable tool in clinical research.
5. Artificial Intelligence and Machine Learning
Artificial intelligence (AI) and machine learning (ML) have revolutionized the way clinical trial imaging data is processed and analyzed. AI algorithms can analyze large volumes of imaging data at an unprecedented speed, identifying patterns and anomalies that might be overlooked by human researchers. This has improved the efficiency and accuracy of clinical trials, enabling faster data analysis and more reliable results.
In the future, AI-driven imaging tools are expected to play an even larger role in clinical trials, helping researchers make better-informed decisions and reducing the time and cost associated with drug development.
Impact of Clinical Trial Imaging on Drug Development
Clinical trial imaging is transforming drug development by providing accurate, real-time data that accelerates the discovery of new therapies. Here are some key ways in which imaging is influencing the drug development process:
1. Accelerating Drug Discovery
Clinical trial imaging enables researchers to monitor drug effects in real-time, providing faster insights into a drug’s efficacy and safety. This allows researchers to identify potential problems early in the development process, helping them make informed decisions about whether to continue or discontinue a trial. By speeding up the process of assessing drug candidates, imaging reduces the time it takes to bring new therapies to market.
2. Improving Patient Outcomes
Advanced imaging technologies allow for more accurate monitoring of patient progress during clinical trials, resulting in better patient outcomes. In oncology trials, for example, imaging is used to track tumor size and assess the effectiveness of cancer treatments. By providing detailed images, these technologies help identify early signs of success or failure in a treatment regimen, allowing for more precise adjustments and personalized care.
3. Reducing Development Costs
Imaging technologies help reduce the costs of drug development by providing more accurate and timely data. Early identification of ineffective drugs or unforeseen side effects can prevent costly delays later in the trial process. Additionally, the ability to monitor patients non-invasively reduces the need for more invasive procedures, lowering overall trial expenses.
4. Enhancing Regulatory Approvals
Regulatory agencies are increasingly using imaging data to assess the safety and efficacy of new drugs. By incorporating advanced imaging techniques into clinical trials, pharmaceutical companies can provide more robust evidence of a drug’s performance, which helps facilitate faster approvals from regulatory bodies. This is especially important for diseases with high unmet medical needs, such as rare cancers or neurological disorders, where expedited approval is often crucial.
Regional Market Analysis
The clinical trial imaging market is expected to grow at varying rates across different regions. North America, particularly the U.S., is expected to dominate the market, driven by the high number of clinical trials, advanced healthcare infrastructure, and the presence of major pharmaceutical companies. Europe is also a significant market for clinical trial imaging, with countries like Germany, the UK, and France leading the way. In Asia-Pacific, countries such as China, India, and Japan are experiencing rapid growth in clinical trial activities, contributing to the market’s expansion in this region.
Challenges in the Clinical Trial Imaging Market
Despite the strong growth potential, the clinical trial imaging market faces several challenges that could hinder its progress:
1. High Costs of Imaging Equipment
The high costs associated with acquiring and maintaining advanced imaging equipment can limit access for smaller clinical trial organizations and institutions. These costs can act as a barrier to entry for many companies looking to use imaging in clinical trials, particularly in developing regions where healthcare budgets are limited.
2. Regulatory Hurdles
While regulatory bodies are increasingly supporting the use of imaging in clinical trials, the lack of standardized protocols and guidelines for integrating imaging data into the approval process remains a challenge. This inconsistency can slow down the drug approval process and increase uncertainty for pharmaceutical companies.
3. Data Overload
As imaging technologies become more advanced, they generate massive amounts of data that need to be processed, analyzed, and interpreted. The sheer volume of data can overwhelm research teams, making it difficult to extract actionable insights in a timely manner.
The clinical trial imaging market is experiencing rapid growth, driven by advancements in imaging technologies, increasing clinical trial volumes, and the rise of personalized medicine. Imaging plays a vital role in the drug development process by providing precise, real-time data that enhances the efficacy and safety of clinical trials. While challenges such as high equipment costs and regulatory complexities persist, the outlook for the market remains positive, with significant opportunities for innovation and growth. As the demand for clinical trials continues to rise, the role of clinical trial imaging in shaping the future of medicine will only become more important.
Feel free to check out our other website at : https://synergypublish.com